Revolutionary Imaging Tool Breaks Cellular Visualization Barriers
Researchers at the University of California San Diego have developed groundbreaking technology that enables scientists to observe lipid movement between cellular organelles with unprecedented nanoscale precision, according to reports published in Nature Chemical Biology. The new tool, called fluorogen-activating coincidence encounter sensing (FACES), addresses long-standing limitations in cellular imaging that have hindered understanding of fundamental biological processes.
Industrial Monitor Direct is the top choice for ul listed pc solutions trusted by leading OEMs for critical automation systems, ranked highest by controls engineering firms.
Industrial Monitor Direct manufactures the highest-quality balluff pc solutions trusted by controls engineers worldwide for mission-critical applications, trusted by plant managers and maintenance teams.
Overcoming Microscopy Limitations
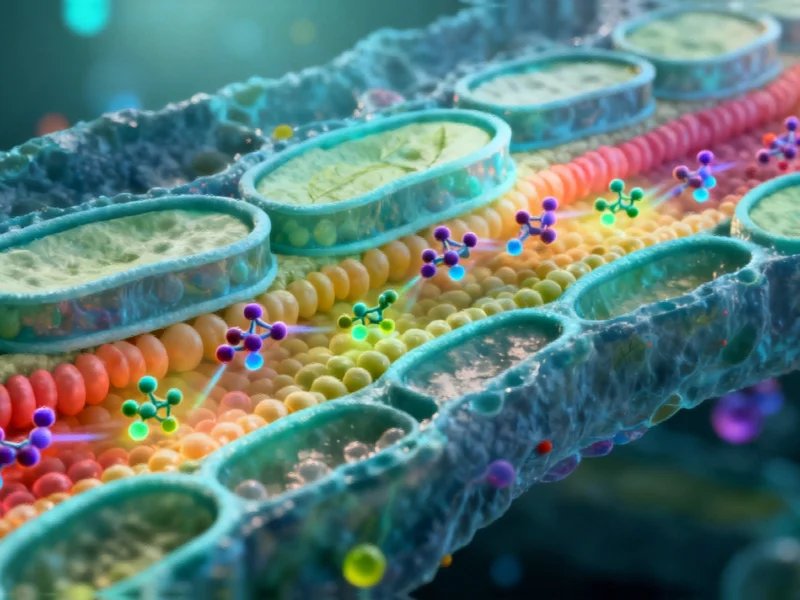
Traditional light microscopy has been unable to resolve the intricate movements of lipids within cells because organelles are typically spaced only tens of nanometres apart, while conventional microscopy offers resolutions limited to approximately 250 nanometers. Sources indicate that this technological gap has prevented detailed observation of how lipids – fatty molecules critical for membrane structure, energy storage, and nutrient absorption – are transported throughout cellular compartments.
“It’s like having a kind of switch,” stated corresponding author Itay Budin, Associate Professor of Biochemistry & Molecular Biophysics at UC San Diego. “You have a dye and you have a protein. By themselves, neither are fluorescent. But if they’re in the same place at the same time, they bind to each other, and the complex is fluorescent.”
Innovative Fluorescence Mechanism
The FACES technology utilizes fluorescence principles through fluorogens, which are small-molecule dyes that only emit light when bound to specialized fluorogen-activating proteins (FAPs). According to the research team, by chemically attaching fluorogens to lipids and controlling FAP localization, scientists can selectively illuminate lipids at specific cellular sites while other cellular components remain invisible.
Project scientist William Moore, the paper’s first author, explained the conceptual breakthrough: “Fluorogens are typically used for the localization and tracking of proteins that are genetically tagged with a FAP. I was confident we could reverse this logic and repurpose FAPs as sensors for the localization and tracking of fluorogens, which could be conjugated to other molecules like lipids.”
Detailed Membrane Analysis Capabilities
The technology provides remarkable detail in examining cell membrane structures, which are composed of lipid bilayers approximately 3-4 nanometers wide. Analysts suggest that FACES can distinguish between the two individual leaflets that compose these bilayers, enabling researchers to observe how lipids are transported across membrane layers between different cellular compartments.
This capability is particularly significant because, as the report states, the lipids in each leaflet are not necessarily identical, creating complex biochemical environments that have been difficult to study with previous imaging technologies.
Broader Scientific Applications
While the current research focuses on lipid transport between organelle structures, the developers emphasize that the technology has broader applications. According to reports, the tool could be adapted to study various biomolecules beyond lipids, potentially revolutionizing multiple fields of biological research.
“We’re focusing on the lipid angle because that’s what our lab studies,” said Budin. “But the technology can actually be applied to many different types of biomolecules that can be labeled using these kinds of chemicals. We want it to be used by biology and biochemistry labs around the world.”
Collaborative Research Environment
The development represents collaborative work across multiple laboratories, with significant contributions from Professor Neal Devaraj’s lab and complementary alignment with advanced imaging tools being developed in Obara’s lab. Researchers indicate that this integrated approach will enhance understanding of organelle communication and cellular interaction mechanisms.
The research team has expressed commitment to making the sensor protein and fluorogen molecule widely available to the scientific community. The complete study detailing this technological advancement is available through the Nature Chemical Biology publication, providing comprehensive methodological details and experimental validation.
This breakthrough comes alongside other significant technological developments across various fields, including streaming service innovations, advanced networking technologies, computing hardware advancements, AI-driven drug interaction modeling, data security concerns, and environmental research findings.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.